Location
Le Bel Institute, 4th floor South
Secretarial and accounting services provided by
Agnès REIFFSTECK
email : reiffste[at]unistra.fr
Phone : +33 (0)3 68 85 14 32
Group's website
Group members
Permanent members
HOURTOULE Maxime
- Maître de Conférences à l'Unistra
- mhourtoule[at]unistra.fr
- +33 (0)3 68 8 51676
- Research team : SOPhy
MIESCH Laurence
- Directrice de Recherche au CNRS
- lmiesch[at]unistra.fr
- +33 (0)3 68 8 5 17 51
- Research team : SOPHY
NOEL DUCHESNEAU Ludovik
- Assistant Ingénieur au CNRS
- noelduchesneau[at]unistra.fr
- +33 (0)3 68 8 5 16 76
- Research team : SOPHY
- Research team : COSyS
Non-permanent members
MENGHINI Marco
- Doctorant
- marco.menghini[at]etu.unistra.fr
- +33 (0)3 68 8 51676
- Research team : SOPhy
SCHITTER Léo
- Doctorant
- leo.schitter[at]etu.unistra.fr
- Research team : SOPhy
SCHUTZ Dorian
- Doctorant
- +33 (0)3 68 8 51676
- Research team : SOPHY
YANG Wenwen
- Doctorante
- wenwen.yang[at]etu.unistra.fr
- +33 (0)3 68 8 51676
- Research team : SOPhy
ZEINOULABADINE Colin
- Doctorant
- czeinoulabadine[at]etu.unistra
- Research team : SOPhy
Research topics
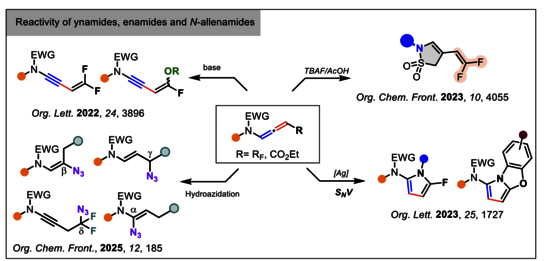
1-Developing new methods in organic synthesis: Reactivity of keto-ynamides
- Study of the reactivity of ynamides, enamides and N-allenamides
2-Phytochemistry
Small molecules that serve as standards for elucidating biosynthetic pathways in plants are being synthesized.
- Oxidized metabolites of jasmonates that are involved plant defence are being constructed as means to identify the enzymes responsible for their formation.
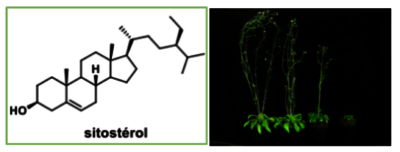
- Sterols are being synthesized to create new probes to understand more clearly the functions of these molecules within the plant cell.
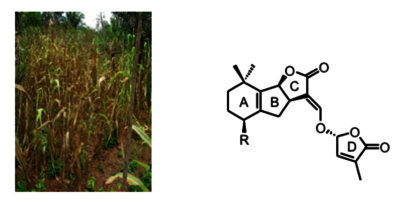
- Synthesis of intermediates of the strigolactone biosynthetic pathway (MeCLA) that promote the growth of parasitic plants. For example, Striga hermonthica, is one of the seven most serious biotic threats to food security, affecting the livelihoods of 100 million people. The goal is to understand this phenomenon to find a way to eradicate this process.
- Synthesis of molecules responsible for aromas in wines in collaboration with INRA.
Description of the research group
SOPhy offers the possibility of internships (IUT, M1-M2 masters, chemistry schools ...) or to prepare a thesis at the University of Strasbourg. The group is recognized for providing excellent training in synthetic organic chemistry.
SOPhy offers research training based on preparative organic chemistry. The thesis is always considered under its double aspect of original scientific work and period of apprenticeship of a profession.
Recent publications
Regio-and Stereoselective Azidation of Activated N-allenamides: an entry to alpha, beta, gamma and delta-amido-azides
D. Schutz, M. Hourtoule and L. Miesch
Org. Chem. Front., 2025, 12, 185 - 191 https://doi.org/10.1039/D4QO01802H
Photocatalyst-free, visible-light-induced regio- and stereoselective synthesis of phosphorylated enamines from N-allenamides via [1,3]-sulfonyl shift at room temperature
J.-D.Guo, F. A. Korsaye, D. Schutz, I. Ciofini and L. Miesch
Chem. Sci., 2024, 15, 17962 -17970, https://doi.org/10.1039/D4SC05190D,
Metal Free Regio – and Stereoselective Semireduction of CF3-Substituted N-Allenamides C. Gommenginger
M. Hourtoule, M. Menghini and L. Miesch
Org. Biomol. Chem., 2024, 22, 940-944 DOI: 10.1039/d3ob01859h
Transition Metal-free Domino Hydroamination/Isomerization/Transamidation Sequence: an Entry to trifluorinated γ-lactams
D. Schutz,C. Gommenginger,B. Moegle, M. Hourtoule, L.- N. Duchesneau, L. Miesch
J. Org. Chem.2024, 89, 10644-10653 https://pubs.acs.org/doi/10.1021/acs.joc.4c00878
OsCYP706C2 diverts rice strigolactone biosynthesis to a noncanonical pathway branch
C. Li, I. Haider, J. Y. Wang, P. Quinodoz, H. G. Suarez Duran, L. Reyes Méndez, R. Horber, V. Fiorilli, C. Votta, L. Lanfranco, S.M. Correia de Lemos, L. Jouffroy, B. Moegle, L. Miesch, A. De Mesmaeker, M. H. Medema, S. Al-Babili, L. Dong, H. J. Bouwmeester
Sci. Adv. 2024, DOI: 10.1126/sciadv.adq3942
TBAF-promoted carbanion mediated sulfonamide cyclization of CF3-substituted N-allenamides an access to fluorinated gamma-sultams
C. Gommenginger, Y. Zheng, D. Maccarone, I.Ciofini and L. Miesch (invited paper)
Org. Chem. Front.2023, 10, 4055 – 4060 https://doi.org/10.1039/D3QO00781B
Evolutionary metabolomics of specialized metabolism diversification in the genus Nicotiana highlights allopolyploidy-mediated innovations in N-acylnornicotine metabolism
D. Elser, D. Pflieger, C. Villette, B. Moegle, L. Miesch, E. Gaquerel
Sci. Adv.,2023, https://www.science.org/doi/10.1126/sciadv.ade8984













![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)