Abstract
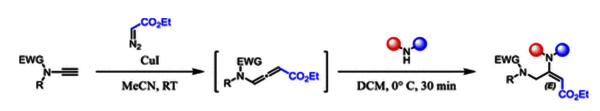
N-Allenamides, substituted by an ester at the γ-position, were obtained through addition of terminal ynamides with ethyl diazoacetate under copper catalysis for the first time. Regio- and stereoselective hydroamination of those activated N-allenamides provided exclusively E-configured captodative enamimes through a one-pot anti-Michael addition. Numerous ynamides as well as various secondary amines were adapted in this process.


![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)