Abstract
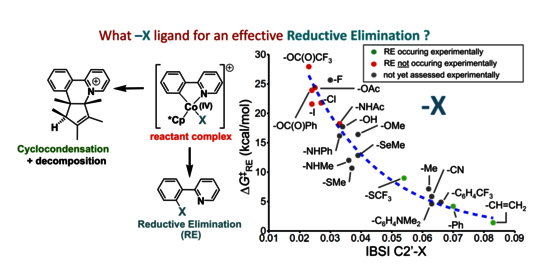
In this joint theoretical and experimental study, the analysis of weak interligand noncovalent interactions within Co(IV) [Cp*Co(phpy)X]+ cobaltacycles (phpy = 2-phenylenepyridine, κC,N) was carried out using the the Independent Gradient Model/Intrinsic Bond Strength Index (IGM/IBSI) method to evaluate the dependency of the catalytically desired reductive elimination pathway (RE) on the nature of the -X ligand. It is shown that the barrier of activation of the RE pathway correlates directly with the IBSI of the X-to-carbanionic chelate's carbon. This correlation suggests that the in silico prediction of which -X ligand is more prone to operate an efficient Cp*Co-catalyzed directed X-functionalization of aromatic C-H bond is at reach. A set of experiments staging various sources of -X ligands supports the theoretical conclusions.


![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)