Abstract
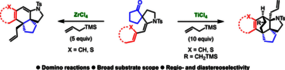
Two unprecedented domino reactions are described, starting from ketospiro-enesulfonamides. By treatment with ZrCl4 and allylsilane, an intramolecular electrophilic aromatic substitution and subsequent allylation is observed. By treatment with TiCl4 and allylsilane, a double enamine-type reaction takes place, thus creating simultaneously four contiguous stereogenic centers diastereoselectively.
Reference
Frédéric Beltran and Laurence Miesch
Tertiary Enamide-Triggered SEAr: Domino Allylation and Enamine-Type Addition
Organic Letters, Published 26th February, 2019 (web) - DOI: 10.1021/acs.orglett.8b03987
Contact chercheur
Laurence Miesch, équipe SOPhy, Institut de Chimie (UMR 7177).

![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)