Abstract
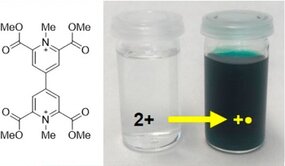
Functionalization of a methylviologen with four methyl ester substituents significantly facilitates the first two reduction steps. The easily generated radical cation shows markedly improved air stability compared to the parent methylviologen, making this derivative of interest in organic electronic applications.
Reference
Mathilde Berville, Jimmy Richard, Monika Stolar, Sylvie Choua, Nolwenn Le Breton, Christophe Gourlaouen, Corinne Boudon, Laurent Ruhlmann, Thomas Baumgartner, Jennifer A. Wytko, and Jean Weiss
A Highly Stable Organic Radical Cation
Org. Lett., 2018, 20 (24), pp 8004–8008 - DOI: 10.1021/acs.orglett.8b03579
Contact chercheur
Jennifer A. Wytko, équipe CLAC, Institut de Chimie (UMR 7177).

![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)