Abstract
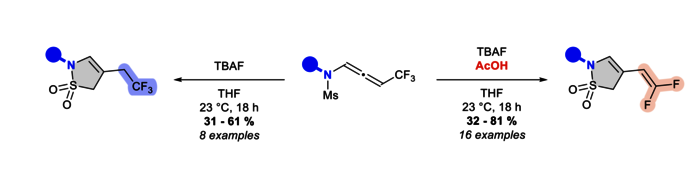
Upon treatment with TBAF, CF3-substituted N-allenamides were transformed into γ-sultams. Cyclic sulfonamides bearing an ene-gem-difluorinated tether could be obtained by addition of acetic acid to the ammonium salt whereas TBAF alone provided the correspondingtrifluorinated ethyl sultams. A combined experimental and computationnal mechanistic study suggested that this transformation involves a 5-endo-dig cyclization on the ene-ynamide generated in situ.


![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)