Abstract
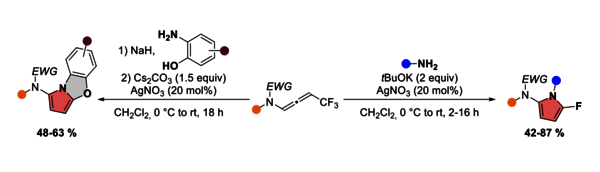
We report herein a domino reaction to construct 2-amido-5-fluoropyrroles from CF3-substituted N-allenamides. The in situ generated gem-difluorinated ene-ynamides derived from CF3-substituted N-allenamides, when subjected to silver catalysis with a primary amine, undergo simultaneous hydroamination of the ynamide moiety followed by a 5-endo-trig addition/β-fluoride elimination sequence, enabling the construction of 2-amido-5-fluoropyrroles. This transformation features excellent functional group compatibility. By employing 2-aminophenols, functionalized benzo-oxazoles were produced.


![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)