Abstract
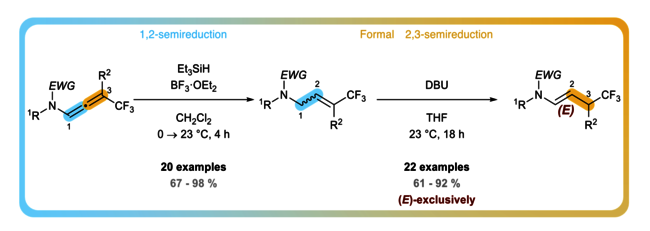
We developed a chemoselective metal-free access for the 1,2- and 2,3-semireduction of CF3-N-allenamides. The enamide functionality of CF3-substituted N-allenamides could be efficiently reduced by Et3SiH/BF3·OEt2 in total regioselectivity and good stereoselectivity, whereas DBU promoted the isomerization of the resulting allyl amide leading exclusively to the E-configurated enamide.


![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)