Abstract
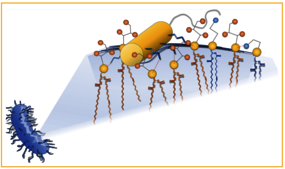
Cathelicidin-BF (CatBF) is a LL-37 homologous antimicrobial peptide (AMP) isolated from Bungarus fasciatus with an exceptional portfolio of antimicrobial, antiviral, antifungal, and anticancer activities. Contrary to many AMPs, it showed a good pharmacological profile with a half-life of at least 1 h in serum and efficacy against bacterial infections in mice. To evaluate its potential against resistant nosocomial infections, we assessed its activity against 81 clinically relevant resistant bacterial isolates. CatBF exhibited minimum inhibitory concentrations (MICs) as low as 0.5 μM against carbapenem-resistant Acinetobacter baumannii, Klebsiella pneumoniae, and Escherichia coli. Its wide-ranging activity, unaffected by resistance mechanisms or Gram phenotype, prompted us to investigate its molecular mode of action. NMR spectroscopy, paramagnetic probes, and molecular dynamics (MD) simulations were employed to define its structure, penetration depth, and orientation in various membrane models, including micelles, bicelles, oriented bilayers, and vesicles. We found that CatBF’s potent activity relies on its strong charge, allowing membrane neutralization at low peptide/lipid ratios and selective recruitment of charged phospholipids. At higher concentrations, a change in peptide orientation reveals membrane invagination and the formation of transient pores possibly leading to bacterial death. Our findings highlight the potential of CatBF as a model for developing resistance-independent agents to combat multidrug-resistant (MDR) bacterial infections.
Reference
Cathelicidin-BF: A Potent Antimicrobial Peptide Leveraging Charge and Phospholipid Recruitment against Multidrug-Resistant Clinical Bacterial Isolates
Evgeniy Salnikov, Morgane Adélaïde, Francisco Ramos-Martín, Ahmad Saad, Jennifer Schauer, Martina Cremanns, Mariam Rima, Christopher Aisenbrey, Saoussen Oueslati, Thierry Naas, Niels Pfennigwerth, Söeren Gatermann, Catherine Sarazin, Burkhard Bechinger, and Nicola D’Amelio.
Journal of the American Chemical Society, 2025 147 (13), 11199-11215 - DOI: 10.1021/jacs.4c17821
Contact
Burkahrd Bechinger (team Biophysique des membranes et RMN), Institut de chimie de Strasbourg, UMR 7177.

![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)