Abstract
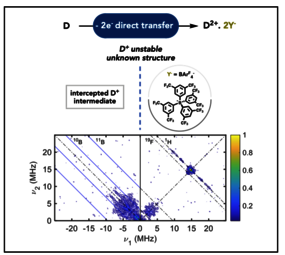
Cooperative multielectron transfer is central to electron storage strategies and small-molecule activation and involves a thermodynamically favored second electron transfer occurring after the first electron transfer. This is the trademark of molecular systems with inverted redox potentials, but it is counterintuitive from an energetic point of view, and the nature of the transient intermediates involved has remained elusive. Using a nickel complex with inverted redox potentials, we show that a weakly coordinating anion (WCA), such as BArF4– ([B(C6H3(CF3)2)4]−), can act as a kinetic trap for the second electron to be transferred. Combined electrochemistry, UV–visible spectroscopy, advanced EPR studies, and theoretical calculations support its existence as a stabilized, confined unpaired electron, close to the concept of a solvated electron. This work sheds light on the nature of energized intermediates in cooperative electron transfer and the possibility of influencing that process through counterion effects.
Reference
Arrested cooperative electron transfer by stabilization of a long-lived confined unpaired electron
Cheriehan Hessin, Mokhtar Ben Ghanem, Nolwenn Le Breton, Sylvie Choua, Aurélien Moncomble, Laurence Grimaud, Hervé Vezin, Marine Desage-El Murr
JACS Au, first published : August 16, 2025 – DOI : https://pubs.acs.org/doi/10.1021/jacsau.5c00484
Contact
Marine Desage-El Murr, team OMECA, Institut de Chimie de Strasbourg, UMR 7177.

![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)