Abstract
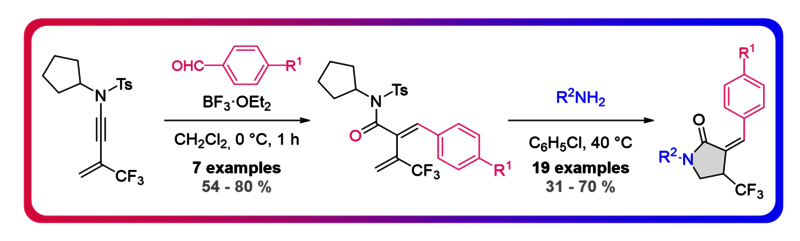
A method for the construction of trifluorinated-5-methylenepyrrolidinone is reported. This strategy combines an acid-catalyzed two-carbon homologation process between ynamides and aldehydes, providing CF3-substituted dienes followed by a metal-free domino hydroamination/isomerization/transamidation sequence, delivering trifluorinated-5-methylenepyrrolidinone stereoselectively.


![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)