Abstract
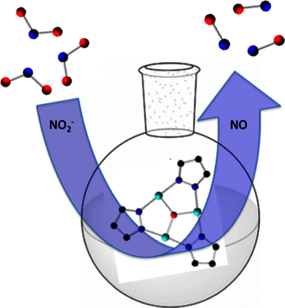
Two trinuclear CuII pyrazolato complexes with a Cu3(μ3-E)-core (E = O2– or OH–) and terminal nitrite ligands in two coordination modes were characterized crystallographically, spectroscopically, and electrochemically. One-electron oxidation of the μ3-O species produces a delocalized, mixed-valent, formally CuII2CuIII-nitrite, but no nitrate. In contrast, under reducing conditions—addition of PhSH as an electron and proton donor—both complexes mediate the reduction of nitrite, releasing NO.
Reference
Kaige Shi, Logesh Mathivathanan, Athanassios K. Boudalis, Philippe Turek, Indranil Chakraborty, Raphael G. Raptis
Nitrite Reduction by Trinuclear Copper Pyrazolate Complexes: An Example of a Catalytic, Synthetic Polynuclear NO Releasing System
Inorg. Chem., 2019, 58 , 11, 7537-7544 - DOI: https://doi.org/10.1021/acs.inorgchem.9b00748
Contact chercheur
Athanassios K. Boudalis (https://pomam.chimie.unistra.fr/aboudalis/), POMAM, Institut de Chimie (UMR 7177).

![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)