Abstract
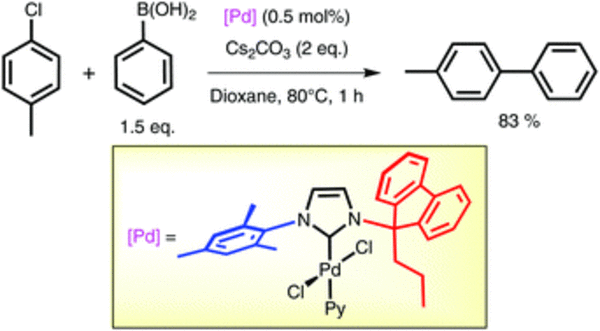
A series of new Pd–PEPPSI complexes containing imidazolylidene ligands with a mixed 9-alkyl-9- fluorenyl/aryl N,N'-substitution pattern have been synthesised. Single crystal X-ray diffraction studies were carried out for four complexes, which revealed that the N-heterocyclic carbene ligands display a semi- open, unsymmetrical space occupancy about the metal. Despite their particular unsymmetrical shape, the new complexes were found to perform as well in Suzuki–Miyaura cross coupling (dioxane, 80 °C) as previously reported, highly active analogues bearing two sterically protecting 9-alkylfluorenyl substituents. They were further found to be considerably more active than the standard Pd–PEPPSI complexes [PdCl2IMes(pyridine)] and [PdCl2IPr(pyridine)]. Surprisingly, unlike the latter, the unsymmetrical complexes of this study were practically inactive in isopropanol at 80 °C. Under these conditions the complexes appear to decompose with formation of non-stabilised nanoparticles.

![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)