Abstract
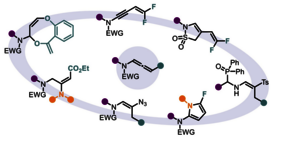
Over the last few decades, allenes have evolved from objects of curiosity to central elements of organic synthesis. Among them, N-allenamides, allenes featuring an electron-withdrawing group on the nitrogen, stand out for their great stability and versatility. Their unique reactivity has made them valuable platforms for numerous chemical transformations. Their interest is reinforced by their ability to efficiently lead to multisubstituted enamides, motifs present in many bioactive natural products but notoriously challenging to synthesize with high regio- and stereocontrol. Driven by our deep interest in the reactivity of activated N-allenamides, particularly those with fluorinated units, we present here new transformations leading to the construction of heterocyclic fluorinated structures and complex enamides, two motifs with significant relevance in medicinal chemistry. the involvement of a carbanionic intermediate in the IspG mechanism.
Reference
Expanding heterocyclic chemical space through the reactivity of N-allenamides
Dorian Schutz and Laurence Miesch
Comptes Rendus. Chimie, 2025, 28, 789-804 – DOI : 10.5802/crchim.414
Contact
Laurence Miesch, team SOPhy, Institut de Chimie de Strasbourg (UMR 7177).


![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)