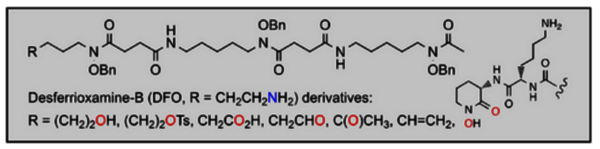
Abstract
Promising results in preclinical diagnosis based on 89Zr-immunoPET have fostered the development of efficient chelators for this tetravalent metal. Leads in this area are octadentate ligands obtained by extension of the desferrioxamine B trihydroxamic acid DFO with a fourth bidentate ligand. In the approach reported here, the latter is a natural 6-membered cyclic hydroxamic acid deriving from (L)-ornithine. Its coupling to DFO via an (L)-lysine spacer required that the genuine amine function of the DFO terminus be changed to a carboxylic acid. Such a requirement prompted us to explore short sequences of chemical transformations that would challenge total syntheses leading to the same products. As a matter of fact, the target C-terminal DFO analogue was obtained in benzyl-protected form in three steps from commercially available DFO in 16% overall yield. Our short-step approach allowed us to implement other functionalities without DFO extension: -OH, -OTs, -CHO, -C(O)CH3, and -CH═CH2.
Graphical Abstract
Functional group transformations were performed at the pendent amine residue of desferrioxamine B (DFO), the commercial siderophore used in various medical applications, including iron decorporation, antibiotic development, and molecular imaging. Our results will broaden the scope of reactions, so far restricted to amide bond formation, for the functionalization of DFO with, for example, a chromophore, an additional ligand, a bioconjugable spacer.

![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)