Abstract
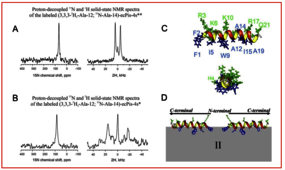
Here we present studies of the structure and membrane interactions of ecPis-4 s, a new antimicrobial peptide from the piscidin family, which shows a wide-range of potential biotechnological applications. In order to understand the mode of action ecPis-4 s, the peptide was chemically synthesized and structural investigations in the presence of anionic POPC:POPG (3:1, mol:mol) membrane and SDS micelles were performed. CD spectroscopy demonstrated that ecPis-4 s has a high content of helical structure in both membrane mimetic media, which is in line with solution NMR spectroscopy that revealed an amphipathic helical conformation throughout the entire peptide chain. Solid-state NMR experiments of ecPis-4 s selectively labeled with 15N/2H and reconstituted into uniaxially oriented POPC:POPG membranes revealed an ideal partition of hydrophilic and hydrophobic residues within the bilayer interface. The peptide aligns in parallel to the membrane surface, a topology stabilized by aromatic side-chain interactions of the Phe-1, Phe-2 and Trp-9 with the phospholipids. 2H NMR experiments using deuterated lipids revealed that anionic lipid accumulates in the vicinity of the cationic peptide upon peptide-membrane binding.
Reference
Elucidating the conformational behavior and membrane-destabilizing capability of the antimicrobial peptide ecPis-4s
K.R. de Souza, L.O. Nunes, E.S. Salnikov, H.M. Mundim, V.H.O. Munhoz, L.M. Li˜ Ao, C. Aisenbrey, J.M. Resende, B. Bechinger, R.M. Verly.
Biophysical Chemistry, 317 (2025) 107353 – DOI : https://doi.org/10.1016/j.bpc.2024.107353
Contact
Burkahrd Bechinger (team Biophysique des membranes et RMN), Institut de chimie de Strasbourg, UMR 7177.

![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)