Abstract
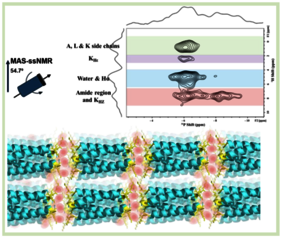
Vectofusin (VF) is a histidine-rich amphipathic peptide designed to enhance lentiviral transduction for gene therapeutic applications, where its assembly into fibrils requires polyvalent anions. In this study, we used solid-state NMR, transmission electron microscopy, and titration experiments to investigate the peptide’s phosphate-driven supramolecular assembly. A VF variant lacking two lysines (V2K) was used to further assess the role of charge in these assemblies. Our results show that VF-pyrophosphate self-assembles into ordered, raft-like sheet structures. NMR confirmed that VF-pyrophosphate aggregates maintain an α-helical conformation, with distinct phosphate populations, one of which closely interacts with lysine residues. In contrast, the V2K variant showed weaker pyrophosphate interactions, highlighting the importance of electrostatic contacts in assembly. Based on these findings, we propose a model in which phosphates act as electrostatic glue, linking peptides via their lysine side chains. These insights support the design of new self-assembling biomaterials and improve understanding of phosphate–polypeptide interactions in biological processes.

![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)