Abstract
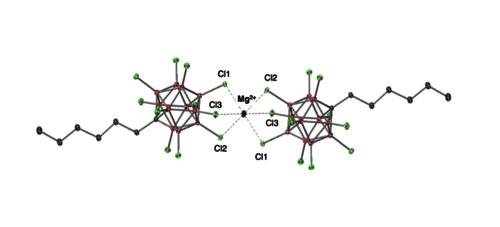
The first soluble and stable Mg2+ dication stabilized only by weakly coordinating and chemically robust carborate anions [HexCB11Cl11]− is described. Mg[HexCB11Cl11]2 (1), prepared by reaction of Mg(nBu)2 with 2 equiv of [Ph3C][HexCB11Cl11], consists, in the solid state, of a central Mg2+ surrounded by two [HexCB11Cl11]− anions. In solution, experimental and classical molecular dynamics simulations (cMD) agree with cation/anion association being retained, reflecting the high electrophilicity of the Mg center. Yet, reflecting only weak anion/cation interactions, species 1 polymerizes 1-hexene and coordinates alkynes. However, 1 displays no reaction with HSiEt3 at room temperature, consistent with a low hydridicity of the hard (HSAB) Mg2+ center. Contrasting with 1 (FIA = 264 kJ mol−1; FIA: Fluoride Ion Affinity), salt Mg[(nBu)3NB12H4Cl7]2 (2), incorporating the more basic ammoniododecaborate [(nBu)3NB12H4Cl7]− anion, is significantly less Lewis acidic (FIA = 214.7 kJ mol−1) and unreactive with alkenes and alkynes. Salt 1 effectively catalyzes alkene/alkyne hydrosilylation via an initial alkene/alkyne coordination/initiation, as suggested by experimental and computational data. It also efficiently catalyzes (with a catalyst loading down to 0.1 mol%) the hydrosilylation of CO2 to CH4 in the presence of HSiEt3. Salt 1 smoothly promotes the catalytic transfer hydrogenation of 1,1-diphenylethylene, and it is also an active imine hydrogenation catalyst in the presence of H2.
Graphical Abstract
Stable Mg2+ dication stabilized only by weakly coordinating carborates was first prepared and structurally characterized as a soluble salt in organic solvents. Combined experimental and theoretical data provided valuable insights on its behavior/reactivity in solution. All data agree with a strong Lewis acidic Mg2+ center, readily coordinating alkenes and alkynes and effectively catalyzing alkene/alkyne hydrosilylation and imine hydrogenation.

![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)