Abstract
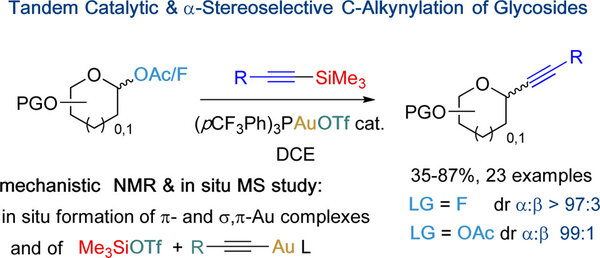
Analogous to O-glycosides, C-glycosides are natural products exhibiting various bioactivities. Alkynyl C-glycosides represent important key intermediates toward more complex derivatives; however, a convenient access through a single catalytic and highly stereocontrolled step remains an important and only partially solved challenge. Here, a mechanistically designed gold(I)-catalyzed silyl-assisted efficient and highly α-stereoselective process is reported. The postulated mechanism has been ascertained by combining 1H, 31P and VT NMR and in situ MS experiments.


![[Translate to English:]](/websites/_processed_/0/4/csm_signature-unistra_fee3442f1d.png)
![[Translate to English:]](/websites/_processed_/0/e/csm_logo-cnrs_c0f610620b.png)
![[Translate to English:]](/websites/_processed_/9/4/csm_logo-fondation-lehn_24043a5484.png)