Abstract
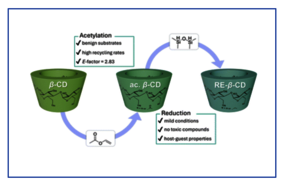
Cyclodextrins (CDs) are bio-based and biodegradable oligosaccharides, enzymatically derived from starch, offering low toxicity and renewability, thus representing an interesting renewable feedstock. While chemical modification allows for precise tailoring of their properties, greater emphasis on sustainability aspects of such processes, for instance a reduction of the produced waste and avoidance of toxic substances, should be aimed for. This work thus focuses on a more sustainable and efficient two-step synthetic alternative approach for the alkylation of b-cyclodextrin (b-CD), especially avoiding the typical use of highly toxic alkylating agents. Transesterification using vinyl acetate facilitated a more sustainable acetylation of b-CD, resulting in an E-factor of 2.83 for the overall process. In a subsequent step, the acetylated b-CDs were successfully reduced under mild conditions to ethylated b-CDs (RE-b-CDs) using near-stoichiometric amounts of TMDS and 4 mol% GaBr3 per ester group, whereby also new mechanistic insights were gained. Notably, anisole was introduced as a more benign, bioavailable alternative to the traditionally used hazardous solvents (e.g. dichloromethane, chloroform). Herein, we present the synthesized RE-b-CDs, which exhibited properties comparable to methylated analogues, including solubility (H2O, MeOH, EtOH, DMSO), thermal stability (TGA/DSC), and host–guest interactions, as confirmed by 2D DOSY and ROESY NMR spectroscopy. Overall, this study establishes a more environmentally friendly access to alkylated b-CDs without compromising characteristic properties of conventionally synthesized methylated CDs, offering a generally more sustainable alternative approach.
Reference
A more sustainable two-step synthesis of alkylated b-cyclodextrin via acetylation and GaBr3-catalyzed reduction
L. J. Bartlewski, P. Conen, Q. Y. Cai, M. Bürk, D. Armspach, M. A. R. Meier
RSC Sustain. 2025, 3, 4126-4136 – DOI : https://doi.org/10.1039/D5SU00590F
Contact
Dominique Armspach, team ECMC, Institut de Chimie de Strasbourg, UMR 7177.