Abstract
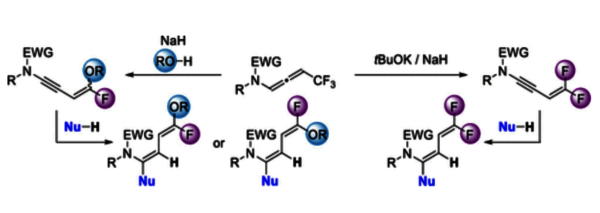
The first synthesis of gem-difluorinated ene–ynamides is presented via deprotonation of trifluoromethylated N-allenamides and δ extrusion of fluorine. These highly reactive building blocks, owing to their dual functional groups, offer a unique entry to difluorinated dienes and to stereodefined, monofluoro-substituted dienes. Stereoselective addition to the ynamide moiety led to difluorinated dienes. A stereocontrolled domino δ elimination reaction followed by an addition/elimination sequence from trifluoromethylated N-allenamides provided exclusively stereodefined monofluorinated ene–ynamides.