ABSTRACT
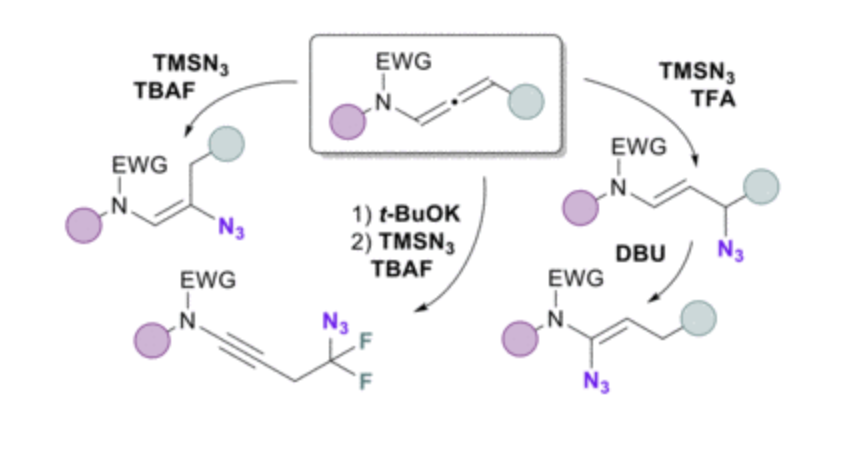
A totally controlled regiodivergent azidation of activated N-allenamides is presented. Using TMSN3/TBAF, β-azidation of N-allenamides occurs exclusively, yielding vinyl azides. Conversely, employing a TFA/TMSN3 mixture results solely in the formation of γ-azides. A subsequent formal Winstein rearrangement of the latter with DBU produces α-amido vinyl azides. Additionally, δ-difluorinated azides featuring an ynamide are selectively synthesized from ene-ynamides. The practical applicability of these transformations is demonstrated through the formation of cyanide derivatives, trifluoromethyl ketones and primary enamines.