Abstract
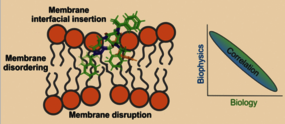
Using antimicrobial peptides as a template, triazolium-based peptoids were designed with strong and selective antibacterial activities. To probe their mechanism, eight distinct peptoids were investigated using biophysical methods with lipid bilayers modeling bacterial or eukaryotic membranes. Calcein leakage experiments closely parallel antibacterial assays testing activities against Gram-negative or Gram-positive bacteria and toxicity for human red blood cells. This excellent correlation shows that the membrane-association underlies these peptoids’ biological activities. While circular dichroism spectroscopy confirms their designed PPI (polyproline I) helical fold, fluorescence assays quantitatively evaluate membrane association and indicate localization at the membrane interface. In the presence of peptoids, a significant reduction in lipid order parameters is observed by solid-state NMR spectroscopy. Collectively, these findings support a membrane-mediated mechanism of action for the triazolium-based peptoids similar to that for linear cationic antimicrobial peptides. Furthermore, the physicochemical and structural features of the peptoids explain their different degrees of biological activities.
Reference
Mechanism of Action and Membrane Interactions of Antibacterial Quaternized Triazolium Peptoids
Kathakali De, Cassandra Guerinot, Nicolas Charbonnel, Allison Faure, Jérôme Josse, Christopher Aisenbrey, Sophie Faure, and Burkhard Bechinger
J. Med. Chem. 2025, 68, 24, 26206–26217 – DOI : https://doi.org/10.1021/acs.jmedchem.5c02254
Contact
Burkahrd Bechinger (team Biophysique des membranes et RMN), Institut de chimie de Strasbourg, UMR 7177.