Abstract
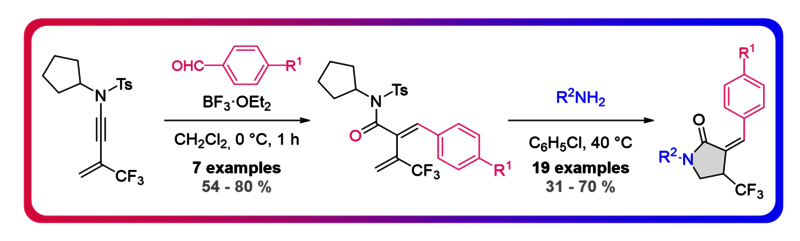
A method for the construction of trifluorinated-5-methylenepyrrolidinone is reported. This strategy combines an acid-catalyzed two-carbon homologation process between ynamides and aldehydes, providing CF3-substituted dienes followed by a metal-free domino hydroamination/isomerization/transamidation sequence, delivering trifluorinated-5-methylenepyrrolidinone stereoselectively.