Abstract
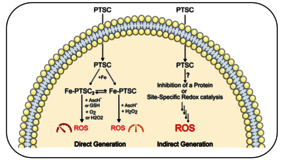
α-N-Pyridylthiosemicarbazones (PTSC) are anticancer agents that can induce oxidative stress in cells, likely through interactions with metal ions. Redox-active Cu and Fe bind strongly to PTSC, forming complexes Cu-PTSC and Fe-PTSC2. These complexes have been proposed to directly catalyze the formation of reactive oxygen species (ROS) and deplete key cellular reductants, thereby exerting oxidative stress. Alternatively, oxidative stress could also arise indirectly through interactions with other cellular targets. Evaluating catalytic rates could help distinguish direct from indirect mechanisms, as ROS production should outpace antioxidant defenses. In this respect, the catalytic activity of the Fe complexes of two PTSCs, Triapine (3AP) and Dp44mT, with the two most abundant reducing agents, ascorbate and glutathione, was evaluated under aerobic conditions. Fe-3AP2 and Fe-Dp44mT2 showed very low catalytic activity in depleting GSH/ascorbate and producing ROS (<4 turnovers per hour). Higher activity appeared with H2O2 and ascorbate, but only for 1:1 Fe-PTSC complexes, not 1:2 Fe-PTSC2. Competition assays with H2O2-degrading enzyme catalase revealed that Fe-PTSC reacted 3 orders of magnitude slower than the enzyme. Thus, Fe-PTSC and Fe-PTSC2 are unlikely to drive ROS production through a direct mechanism. Instead, an indirect mechanism or a site-specific ROS production appears to be more plausible.
Reference
Low Catalytic Redox Activity of α-N-Pyridylthiosemicarbazone Iron Complexes Suggests an Indirect ROS Generation Mechanism in Their Biological Activity
Bharath Vinjamuri, Christian R. Kowol, and Peter Faller
Inorganic Chemistry, 2025, 64, 40, 20340–20347 – DOI : doi.org/10.1021/acs.inorgchem.5c03520
Contact
Peter Faller, team BCB, Institut de Chimie de Strasbourg, UMR 7177.