Abstract
Imaging extracellular Cu2+ in vivo is important due to its role in physiological and pathological processes. Magnetic resonance imaging is a promising modality for this purpose but developing contrast agents selective for Cu2+ over abundant Zn2+ ions remains a challenge. We synthesized and characterized two novel ligands, DO3A-picG and DO3A-picGH, containing a macrocycle and a pendant arm for Cu2+ complexation inspired from the ATCUN site of human serum albumin. The corresponding Ln3+ complexes were studied in the presence and in the absence of Cu2+ through UV-visible, fluorescence and EPR spectroscopies, as well as relaxivity measurements. These studies show that Gd-DO3A-picG and Gd-DO3A-picGH are non-hydrated complexes, in which the pyridine-amide moiety remains coordinated to Ln3+ even in the presence of Cu2+. While Gd-DO3A-picG does not respond to Cu2+, a sizeable relaxivity increase is observed for Gd-DO3A-picGH. This increase is attributed to an increased second-sphere contribution to the relaxivity, i. e. a higher number of H2O molecules retained in close proximity to Gd3+ through H-bonding. Finally, we show that Gd-DO3A-picGH binds Cu2+ with a μM affinity, and is selective for Cu2+ vs Zn2+.
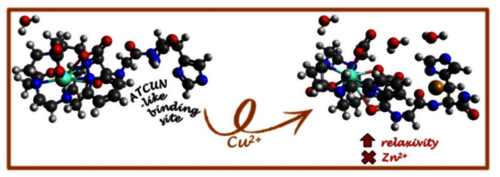
Graphical Abstract
Gd3+ complexes bearing a bioinspired Cu2+-binding site derived from ATCUN are studied as MRI contrast agents. The presence of two amino acids (GH) is necessary to bind Cu2+ and trigger a relaxivity increase. This is due to a higher number of H2O retained close to Gd3+ through H-bonding with Cu2+. Remarkably, the system remains selective for Cu2+ vs Zn2+.