Abstract
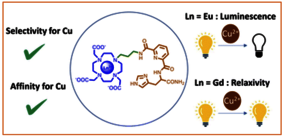
In the pursuit of CuII-responsive MRI contrast agents with enhanced CuII affinity, we report the synthesis and characterization of two LnIII-DO3A-C3AmpicGH complexes (LnIII = GdIII, EuIII), featuring a tetradentate CuII-binding moiety, along with the corresponding mononuclear CuII-C3AmpicGH complex. The chelator coordinates CuII through two amide groups, a pyridine and an imidazole ring. The use of a propyl-amide linker between the LnIII- and CuII-binding moieties allowed an increased separation between the two metal centers. As a result, both LnIII–CuII-DO3A-C3AmpicGH and CuII-C3AmpicGH complexes exhibit the same CuII-affinity (log K = 11–12 at pH 7.4) and good selectivity over competing physiological metal ions, particularly ZnII (up to at least 500 equivalents). While the GdIII complex displayed no relaxivity changes upon CuII binding, the EuIII analogue showed a luminescence turn-off response. Spectroscopic analyses as well as density functional theory (DFT) calculations provide insights into the coordination environments of both metal ions, notably confirming the absence of amide coordination to the LnIII center, even in absence of CuII.
Reference
Lanthanide complexes bearing a bioinspired CuII binding site with picomolar affinity: synthesis, structural, relaxometric and luminescence studies
Katharina Zimmeter, Bertrand Vileno, Agnès Pallier, Carlos Platas-Iglesias, Peter Faller, Célia S. Bonnet, and Angélique Sour
Dalton Transactions, 2025, 54, 15432-15440 – DOI : https://doi.org/10.1039/D5DT01735A
Contacts
Angélique SOUR & Pater FALLER, team BCB, Institut de Chimie de Strasbourg (UMR 7177).
Bertarand VILENO, team POMAM, Institut de Chimie de Strasbourg (UMR 7177).