Abstract
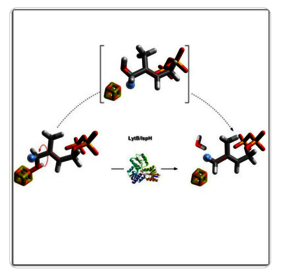
IspH/LytB, an oxygen‐sensitive [4Fe‐4S] enzyme, catalyzes the last step of the methylerythritol phosphate (MEP) pathway, a target for the development of new antimicrobial agents. This metalloenzyme converts (E)‐4‐hydroxy‐3‐methylbut‐2‐en‐1‐yl diphosphate (HMBPP) into the two isoprenoid precursors: isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). Here, the synthesis of (S)‐[4‐2H1]HMBPP and (R)‐[4‐2H1]HMBPP is reported together with a detailed NMR analysis of the products formed after their respective incubation with E. coli IspH/LytB in the presence of the biological reduction system used by E. coli to reduce the [4Fe‐4S] center. (S)‐[4‐2H1]HMBPP was converted into [4‐2H1]DMAPP and (E)‐[4‐2H1]IPP, whereas (R)‐[4‐2H1]HMBPP yielded [4‐2H1]DMAPP and (Z)‐[4‐2H1]IPP, hence providing the direct enzymatic evidence that the mechanism catalyzed by IspH/LytB involves a rotation of the CH2OH group of the substrate to display it away from the [4Fe‐4S].
Reference
Philippe Chaignon, Benoît Eric Petit, Bruno Vincent, Lionel Allouche, and Myriam Seemann
Methylerythritol Phosphate Pathway: Enzymatic Evidence for a Rotation in the LytB/IspH‐Catalyzed Reaction
Chemistry A European Journal, First published: 22 November 2019 - DOI: https://doi.org/10.1002/chem.201904676
Contact chercheurs
Myriam Seemann, équipe CBAT, Institut de Chimie (UMR 7177). Lionel Allouche, Service de RMN de la Fédération de Chimie Le Bel (FR 2010).