Abstract
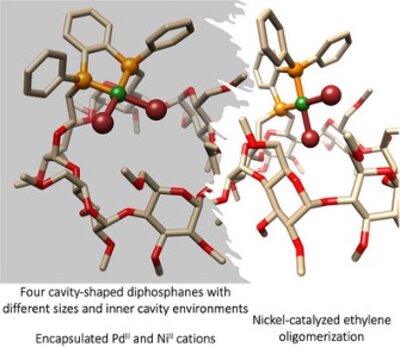
Four cis-chelating diphosphanes derived from cyclodextrins (CDs), each featuring a distinct intracavity environment, compel NiII or PdII metal centers to reside within α- or β-CD cavities. Nickel(II) complexes of these metal-confining ligands act as active catalysts in ethylene oligomerization upon activation with modified methylaluminoxane (MMAO). The size of the cavity and the position of the P2Ni fragment relative to the cavity affect both the activity and selectivity of the reaction. In all instances, 1-butene is the major product (up to 98% C4 products and 90% 1-butene within the C4fraction). Extensive theoretical studies with state-of-the-art methods carried out on the most selective system suggest that the CD cavity restricts isomerization pathways by limiting the mobility of the coordinated olefin in this constrained supramolecular environment, thereby enhancing α-olefin formation.
Graphical Abstract
Four cavity-shaped diphosphanes have been synthesized from both α- and β-cyclodextrins. The metal-confining properties of these ligands were exploited in nickel-catalyzed ethylene oligomerization.