Abstract
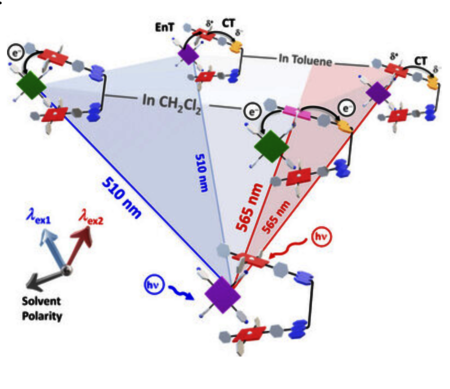
The 1:1 complex of a bis(acridinium-Zn(II)porphyrin) host and a tetrapyridyl free-base porphyrin guest shows a remarkable interplay of energy and electron transfer (eT) processes, finely tuned by the solvent polarity or the selection of the photoexcited component. A more polar environment (CH2Cl2) favors uncommon parallel intramolecular and intermolecular eT processes, while in an apolar solvent (toluene) the intermolecular eT process is hampered. Notably, the apolar environment also enables a rare energy transfer process from the porphyrin guest toward the charge-transfer emissive state of the tweezer host. In total, no less than four different excited states were probed, which can be sketched by an inverted pyramid diagram highlighting directional path selection.
Graphical Abstract
Tuning unusual energy and eT processes in a bis(acridinium-Zn(II) porphyrin)-tetrapyridyl porphyrin host-guest complex.