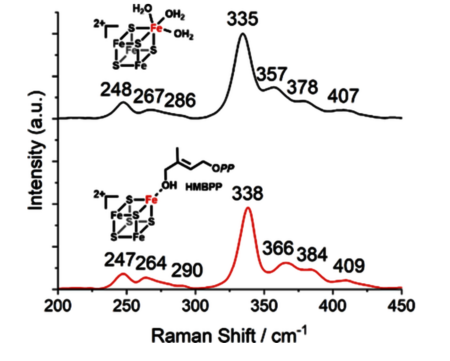
Abstract
IspH is the last enzyme of the methylerythritol phosphate pathway. It catalyzes the reductive dehydroxylation of (E)-4-hydroxy-3-methyl-but-2-en-1-yl diphosphate into isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), which are precursors for the biosynthesis of terpenoids, essential molecules for the survival of all living organisms. This pathway is absent in humans, making it a promising target for drug discovery. Escherichia coli IspH harbors an unusual [4Fe-4S]2+ cluster linked to three conserved cysteines with a unique iron site proposed to be coordinated to three water molecules. Here, the first resonance Raman spectroscopic study of the cluster of IspH in the 2+ oxidation state is reported. Using isotopic labeling with 2H2O and H218O, the bands of the cluster that are sensitive to water coordination or hydrogen bonding are identified. The change of geometry of the cluster upon binding of the substrate, an alkyne diphosphate inhibitor, and the two enzyme products is also analyzed. Distinct binding modes to the cluster may indeed be at the origin of the different distribution of IPP and DMAPP observed during catalysis.