Abstract
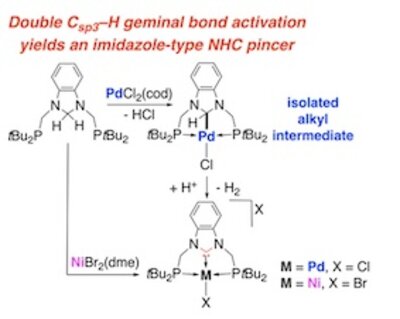
We report the first examples of metal-promoted double geminal activation of C(sp 3 )–H bonds of the N–CH 2 –N moiety in an imidazole-type heterocycle, leading to nickel and palladium NHC complexes under mild conditions. Reaction of the new electron-rich diphosphine 1,3-bis((di- tert -butylphosphaneyl)methyl)-2,3-dihydro-1 H -benzo[ d ]imidazole ( 1 ) with [PdCl 2 (cod)] occurred stepwise, first by single C–H bond activation yielding the alkyl pincer complex [PdCl(PC sp3 H P)] ( 3 ) with two trans phosphane donors and a covalent Pd–C sp3 bond. Activation of the C–H bond of the resulting α-methine C sp3 H–M group occurred subsequently when 3 was reacted with HCl to yield the NHC pincer complex [PdCl(PC NHC P)]Cl ( 2 ). The reaction of 1 with [NiBr 2 (dme)] also afforded a NHC pincer complex, [NiBr(PC NHC P)]Br ( 6 ), but the reactions leading to the double geminal C–H bond activation of the N–CH 2 –N group were too fast to allow identification or isolation of an intermediate analogous to 3 . The determination of six crystal structures, the isolation of reaction intermediates and DFT calculations provided the basis for suggesting the mechanism of the stepwise transformation of a N–CH 2 –N moiety in the N–C NHC –N unit of NHC pincer complexes and explain the key differences observed between the Pd and Ni chemistries.