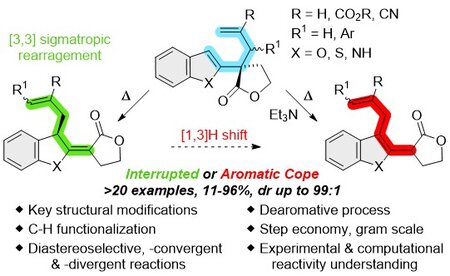
Abstract
The aromatic Cope rearrangement is an elusive transformation that has been the subject of a limited number of investigations compared to those seemingly close analogues, namely the Cope and aromatic Claisen rearrangement. Herein we report our investigations inspired by moderate success observed in the course of pioneering works. By careful experimental and theoretical investigations, we demonstrate that key substitutions on 1,5-hexadiene scaffold allow fruitful transformations. Especially, efficient functionalisation of the heteroaromatic rings results from the aromatic Cope rearrangement, while highly stereoselective interrupted aromatic Cope rearrangements highlight the formation of chiral compounds through a dearomative process.
Graphical Abstract