Abstract
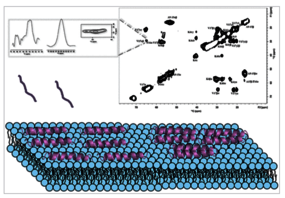
In this study, we present an atomic-level structural investigation of the magainin 2 antimicrobial peptide reconstituted in extended lipid bilayers that closely mimic the composition of bacterial membranes. Using state-of-the-art solid-state NMR spectroscopy, we show that within liquid-crystalline membranes the peptide exhibits site-specific motional regimes, which correlate with its amphipathic character. Peptide-lipid interactions are identified at the polar headgroup region consistent with an in-plane topology also observed by oriented 15N solid-state NMR spectroscopy. While 13C chemical shift analysis reveals α-helical conformations, the NMR line shapes indicate pronounced conformational heterogeneity, which can be explained by the existence of higher order arrangements along the membrane surface. A reduced degree of helicity is observed when the membrane is in the gel phase suggesting more superficial interactions of magainin 2. Notably, our NMR data show that membrane-associated magainin 2 can evolve into amyloid-like β-sheet structures, forming large peptide-lipid aggregates. This behavior occurs only in bacterial and not in mammalian membrane models, paving the way for a new understanding of the role of these supramolecular assemblies in host defense mechanisms, and highlighting a potential relationship between antimicrobial peptides and functional amyloid structures.
Reference
Structure and Dynamics of the Magainin 2 Antimicrobial Peptide in Biomimetic Lipid Bilayers by Solid-State NMR
Ahmad Saad, Jesus Raya, and Burkhard Bechinger.
Biochemistry, first published september 29 (2025) – DOI : 10.1021/acs.biochem.5c00467
Contact
Burkahrd Bechinger (team Biophysique des membranes et RMN), Institut de chimie de Strasbourg, UMR 7177.