Abstract
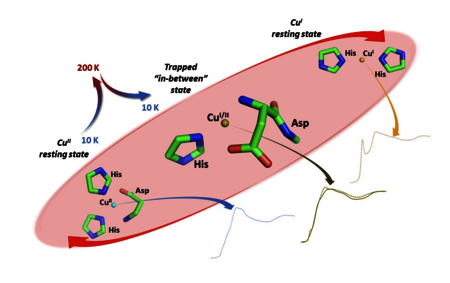
The redox activity of Cu ions bound to the amyloid-β (Aβ) peptide is implicated as a source of oxidative stress in the context of Alzheimer's disease. In order to explain the efficient redox cycling between Cu(II)-Aβ (distorted square pyramidal) and Cu(I)-Aβ (digonal) resting states, the existence of a low-populated "in-between" state, prone to bind Cu in both oxidation states, has been postulated. Here, we exploited the partial X-ray induced photoreduction at 10 K, followed by a thermal relaxation at 200 K, to trap and characterize by X-ray Absorption Spectroscopy (XAS) a partially reduced Cu-Aβ1−16 species different from the resting states. Remarkably, the XAS spectrum is well-fitted by a previously proposed model of the "in-between" state, hence providing the first direct spectroscopic characterization of this intermediate state. The present approach could be used to explore and identify the catalytic intermediates of other relevant metal complexes.