Summary
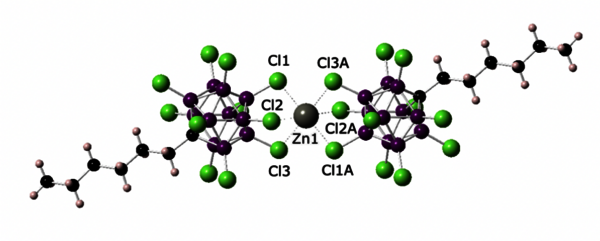
A salt compound containing a formal Zn2+ dication only stabilized by weakly coordinated carborate anions was first structurally characterized and shown to be soluble in low polarity solvents. Based on its reactivity with alkene, alkyne and in hydrosilylation catalysis, it is a strong Lewis acid, possibly the strongest Zn-based Lewis acid to date.