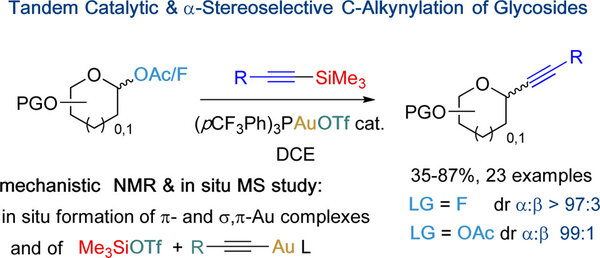
Abstract
Analogous to O-glycosides, C-glycosides are natural products exhibiting various bioactivities. Alkynyl C-glycosides represent important key intermediates toward more complex derivatives; however, a convenient access through a single catalytic and highly stereocontrolled step remains an important and only partially solved challenge. Here, a mechanistically designed gold(I)-catalyzed silyl-assisted efficient and highly α-stereoselective process is reported. The postulated mechanism has been ascertained by combining 1H, 31P and VT NMR and in situ MS experiments.